This is the end of all threads for questions dealing with the nature of tank pressure. After reading this you should be able to answer any questions you may have regarding any aspect of your tank pressure or weight.
First thing I will do is introduce you to my friend, the Ideal Gas Law. This will solve almost ALL of your questions if you know how to use it. It's really cool!
PV=nRT
Where P is the Pressure, V is the Volume, n is the Number of moles, R it the Rate constant, and T is the Temperature in Kelvin. You input four of the five values you have, then solve for the remaining one.
For those of you that are foggy on those days in chemistry class or perhaps have not had the joy of learning the subject yet here is a brief overview of the 5 variables used in the above equation.
Pressure is the force per unit area applied to an object. The one you will probably encounter the most will be PSI or Pressure per Square Inch. You can use this in the equation straight up as PSI, however, for reasons coming up soon, I like to convert to the metric system equivalent which is atmospheres. At ground level the pressure on the earth is 1 atmosphere, or atm for short. To convert between these two values (PSI --> atm) use Google. It is your friend. A typical query might be "4500 psi to atm" and Google will convert it and give you the answer.
Volume is the amount of space an object occupies. This is what the first part of a tank is usually quantified as and it is usually in cubic inches (sometimes seen abbreviated as "ci"), ex. 68/4500 is 68 cubic inches at a pressure of 4500 PSI. In the ideal gas law we use Liters, or L for short. Again, to convert between cubic inches and liters use Google. A typical query might be "68 cubic inches to liters", I have also found you need to type out 'cubic inches' and not just type 'ci' or else Google will not recognize the unit.
Moles (abbreviated "mol") is an amount of something. Anything. It could be paintballs, it could be cars, it could be pixels on your monitor. But in science it represents how many molecules of a specific substance there is, in our case carbon dioxide (CO2) or air. One mole is a very specific number, a very large number. It is an enormous number! It is 6.02*10^23, or 602,000,000,000,000,000,000,000. To give you an idea here's a few examples:
-One mole of inches is 1,616,434 light years, or across our galaxy and back 8 times.
-One mole of seconds is about 19 quadrillion years, 4,240,666 times the age of the earth, or 954,150 times the age of the universe itself.
-One mole of cents could repay the United States National Debt(2004) 86 million times.
-One mole of kilograms is just over 20 times the mass of the earth.
This is usually the variable most people want to solve for first, since after you get the number of molecules of air in your tank you can do many other calculations with it. You can convert moles of any known substance to how much it will weigh. For the purposes of this discussion air is 28.97 grams per mole and carbon dioxide is 44.01 grams per mole. So if I have 3 moles of air I have...(3 mol)(28.97g/mol) = 86.91g of air. You can then convert to how much it weighs in pounds by asking Google: "86.91g to pounds" and it will tell you that is is ~0.192 pounds.
R is the rate constant. This is why I have you convert to metric units because it is an easy number to remember. It is .0821, it does not represent anything other than a multiplier to keep the equation equal. Now, that number will only work as long as the 4 others are mol, atm, L, and Kelvin which we'll get to...now.
Temperature. Now, I know you're thinking "I know what temperature means dude..." but you may not realize what it really is. Temperature is the average energy in each degree of freedom in the particles of a system. The sensation of hot and cold is actually caused by molecules moving faster or slower in the object than in your hand! In metric it is either Celsius (*C) or Kelvin (K). We use Kelvin in this equation because Kelvin will never go to 0 (well it could but that's for another time and place) so we will never have to worry about multiplying by negatives or small numbers since both Celsius and Fahrenheit go through a spectrum of an extreme negative number to a high positive number. We need to know how fast are those molecules in that tank moving because they influence pressure. Average room temperature is 72*F, which is 295 kelvin. Again, converting between Fahrenheit, Kelvin and Celsius should be easy with the help of Google. A typical query might be "72 degrees fahrenheit to kelvin".
PHEW! Now that you understand the terms in the equation you can calculate a lot of cool things! Below are some samples!
How much does the air in my tank weigh? Yes, the air in your tank is matter; it takes up space and has mass. So it does add weight to your tank, let's see how much the air in a 68/4500 weighs in pounds!
1. First convert all known values to metric with the help of Google:
68 (cubic inches) = 1.11 liters
4500 pounds per square inch = 306.2 atm
72 *F = 295 Kelvin
2. Plug the numbers into the equation and solve for the unknown:
(306.2 atm)(1.11 L) = (n)(.0821)(295 K)
n = 14.03 moles of air
3. There it is you successfully solved for moles! You now know how many molecules there are in your tank at a given volume, temperature and pressure!
You can now convert to how much the air weighs easy mode. Simply take the number of moles and multiply it by how much one normal mole of air weighs.
(14.03 mol)(28.97 g/mol) = x
x = 406.45 grams
To convert grams to pounds just use Google:
405.58 grams = 0.894 pounds
How much space would the air in my 68/4500 take up if it were at atmospheric pressure?
1. First convert all known values to metric with the help of Google:
68 (cubic inches) = 1.11 liters
4500 pounds per square inch = 306.2 atm
72 *F = 295 Kelvin
2. Plug the numbers into the equation and solve for the unknown:
(306.2 atm)(1.11 L) = (n)(.0821)(295 K)
n = 14.03 moles of air
3. Solve for atmospheric pressure now that we have moles:
(1 atm)(x L) = (14.03 mol)(.0821)(295 K)
x = 339.8 liters
4. Convert liters to cubic feet with the help of Google:
339.8 liters = 12.00 cubic feet!
How much more air does a 68/4500 hold than a 45/3000?
1. First convert all known values to metric with the help of Google:
68 (cubic inches) = 1.11 liters
45 (cubic inches) = 0.737 liters
4500 pounds per square inch = 306.2 atm
3000 pounds per square inch = 204.14 atm
72 *F = 295 Kelvin
2. Plug the numbers into the equation and solve for the unknown:
(306.2 atm)(1.11 L) = (n)(.0821)(295 K)
n = 14.03 moles of air
(204.14 atm)(0.737 L) = (n)(.0821)(295 K)
n = 6.21 moles of air
3. Subtract to get the difference:
14.03 moles - 6.21 moles = 7.82 moles of air more than the 45/3000 OR ~2.25 times more air!
A full 68/4500 is lighter than a full 48/3000, right?
1. First convert all known values to metric with the help of Google:
68 (cubic inches) = 1.11 liters
48 (cubic inches) = 0.787 liters
4500 pounds per square inch = 306.2 atm
3000 pounds per square inch = 204.1 atm
72 *F = 295 Kelvin
2. Plug the numbers into the equation and solve for the unknown:
(306.2 atm)(1.11 L) = (n)(.0821)(295 K)
n = 14.03 moles of air
(204.1 atm)(0.787 L) = (n)(.0821)(295 K)
n = 6.63 moles of air
3. Calculate weight of the air in addition to the tank:
(14.03 mol)(28.97 g/mol) = 406.45g of air
406.45 grams = 0.896 pounds of air
(6.63 mol)(28.97 g/mol) = 192.07g of air
192.07 grams = 0.423 pounds of air
4. Add to tank weight unfilled:
A ninja 68/4500 weighs 2.5 pounds unfilled.
2.50 lbs + .896 lbs = 3.396 pounds full
A ninja 48/3000 weighs 2.82 pounds unfilled
2.82 lbs + .423 lbs = 3.243 pounds full
So when both tanks are full the 48/3000 is actually lighter than the 68/4500!
What is the cost to contain one mole of air in a aluminum tank rather than a carbon fiber, specifically a 72/3000 vs. a 68/4500?
1. First convert all known values to metric with the help of Google:
68 (cubic inches) = 1.11 liters
72 (cubic inches) = 1.18 liters
4500 pounds per square inch = 306.2 atm
3000 pounds per square inch = 204.14 atm
72 *F = 295 Kelvin
2. Plug the numbers into the equation and solve for the unknown:
(306.2 atm)(1.11 L) = (n)(.0821)(295 K)
n = 14.03 moles of air
(204.14 atm)(1.18 L) = (n)(.0821)(295 K)
n = 9.95 moles of air
3. Do a cost per mole of air:
For the 72/3000 steely:
$70.00/9.95 mol = $7.03/mol
For the 68/4500:
$144.95/14.03 mol = $10.33/mol
So it costs $3.30 more to contain one mole of air in a carbon fiber tank than a steel tank.
How did we come up with this rule of thumb? And how can we predict how many shots our marker will get with any tank at any pressure?
TheLaughingMan, on Sep 30 2008, 07:49 PM, said:
Q: I want an HPA tank but I don't know how to tell how many shots there are, what number matters the ci or the psi?
A: It's actually a combination of the two you multiply the first number by either 10, 15, or 18-20 based on the psi. I'll give a little chart as a visual example.
3000 psi- multiply the cubic inches by 10
4500 psi- multiply the cubic inches by 15
(Also I would like to add that these numbers are not exact as different paintball markers, regs, valves, and bolt efficiency can give you more or less shots, and in some cases you may get a bad fill. So take into account that these may be off by a little bit but shouldn't be off by more than 50 shots either way.)
So basically...
45/3000 = 45x10 = 450 shots
47/3000 = 47x10 = 470 shots
45/4500 = 45x15 = 675 shots
68/3000 = 68x10 = 680 shots
88/3000 = 80x10 = 800 shots
90/3000 = 90x10 = 900 shots
68/4500 = 68x15 = 1020 shots
70/4500 = 70x15 = 1050 shots
88/4500 = 80x15 = 1320 shots
90/4500 = 90x15 = 1350 shots
For CO2...
24 oz = 1200 shots
20 oz = 1000 shots
16 oz = 800 shots
12 oz = 600 shots
9 oz = 450 shots
4 oz = 200 shots
1. First convert all known values to metric with the help of Google(LOTS!):
45 (cubic inches) = 0.737 L
47 (cubic inches) = 0.770 L
68 (cubic inches) = 1.11 L
70 (cubic inches) = 1.15 L
88 (cubic inches) = 1.44 L
90 (cubic inches) = 1.48 L
3000 pounds per square inch = 204.1 atm
4500 pounds per square inch = 306.2 atm
24 ounces = 680.39 grams
20 ounces = 566.99 grams
16 ounces = 453.59 grams
12 ounces = 340.19 grams
9 ounces = 255.15 grams
4 ounces = 113.40 grams
2. Plug the numbers into the equations and solve for the unknowns:
(204.1 atm)(.737 L) = (n)(.0821)(295 K)
n = 6.21 moles of air in a 45/3000
(204.1 atm)(.770 L) = (n)(.0821)(295 K)
n = 6.49 moles of air in a 47/3000
(204.1 atm)(1.11 L) = (n)(.0821)(295 K)
n = 9.35 moles of air in a 68/3000
(204.1 atm)(1.44 L) = (n)(.0821)(295 K)
n = 12.14 moles of air in a 88/3000
(204.1 atm)(1.48 L) = (n)(.0821)(295 K)
n = 12.47 moles of air in a 90/3000
(306.2 atm)(.737 L) = (n)(.0821)(295 K)
n = 9.32 moles of air in a 45/4500
(306.2 atm)(.77 L) = (n)(.0821)(295 K)
n = 9.73 moles of air in a 47/4500
(306.2 atm)(1.11 L) = (n)(.0821)(295 K)
n = 14.03 moles of air in a 68/4500
(306.2 atm)(1.15 L) = (n)(.0821)(295 K)
n = 14.54 moles of air in a 70/4500
(306.2 atm)(1.44 L) = (n)(.0821)(295 K)
n = 18.21 moles of air in a 88/4500
(306.2 atm)(1.48 L) = (n)(.0821)(295 K)
n = 18.71 moles of air in a 90/4500
680.39 grams CO2 / (44.01 g/mol) = 15.46 moles of CO2 in a 24 ounce
566.99 grams CO2 / (44.01 g/mol) = 12.88 moles of CO2 in a 20 ounce
453.59 grams CO2 / (44.01 g/mol) = 10.31 moles of CO2 in a 16 ounce
340.19 grams CO2 / (44.01 g/mol) = 7.73 moles of CO2 in a 12 ounce
255.15 grams CO2 / (44.01 g/mol) = 5.80 moles of CO2 in a 9 ounce
113.40 grams CO2 / (44.01 g/mol) = 2.58 moles of CO2 in a 4 ounce
3. Find the efficiency in moles per shot:
45/3000 = 450 shots
6.21 moles / 450 shots = .0138 moles of air per paintball fired
47/3000 = 470 shots
6.49 moles / 470 shots = .0138 moles of air per paintball fired
45/4500 = 675 shots
9.32 moles / 675 shots = .0138 moles of air per paintball fired
68/3000 = 680 shots
9.35 moles / 680 shots = .0138 moles of air per paintball fired
88/3000 = 800 shots
12.14 moles / 800 shots = .0152 moles of air per paintball fired
90/3000 = 900 shots
12.47 moles / 900 shots = .0139 moles of air per paintball fired
68/4500 = 1020 shots
14.03 moles / 1020 shots = .0138 moles of air per paintball fired
70/4500 = 1050 shots
14.54 moles / 1050 shots = .0138 moles of air per paintball fired
88/4500 = 1320 shots
18.21 moles / 1320 shots = .0138 moles of air per paintball fired
90/4500 = 1350 shots
18.71 moles / 1350 shots = .0139 moles of air per paintball fired
24 oz = 1200 shots
15.46 moles / 1200 shots = .0129 moles of CO2 per paintball fired
20 oz = 1000 shots
12.88 moles / 1000 shots = .0129 moles of CO2 per paintball fired
16 oz = 800 shots
10.31 moles / 800 shots = .0129 moles of CO2 per paintball fired
12 oz = 600 shots
7.73 moles / 600 shots = .0129 moles of CO2 per paintball fired
9 oz = 450 shots
5.80 moles / 450 shots = .0129 moles of CO2 per paintball fired
4 oz = 200 shots
2.58 moles / 200 shots = .0129 moles of CO2 per paintball fired
So now we see that the x10 or x15 rule has an assumed efficiency factor of .138 moles per paintball when using HPA and .0129 moles per paintball when using CO2. Knowing this you can find out how many shots you would get at any pressure, volume, or temperature! Additionally, you can figure out the efficiency factor for your marker and know with great precision how many shots you would get on any HPA tank.
I believe TheLaughingMan had a question?
TheLaughingMan, on Sep 30 2008, 07:49 PM, said:
5000 psi- multiply the cubic inches by 18-20 (If someone has an exact number I'd appreciate it)
Well now we know the efficiency factor we are using for HPA all we have to do is...
1. First convert all known values to metric with the help of Google:
45 (cubic inches) = 0.737 L
5000 pounds per square inch = 340.23 atm
2. PLUG AND CHUG!!!
(340.23 atm)(.737 L) = (n)(.0821)(295 K)
n = 10.35 moles of air in a 45/5000
(10.35 moles) / (.0138 shots/mol) = 750 shots on a 45/5000
3. Find out what the multiplier is:
(45 cubic inches)(x) = 750
x = 16.67
And there is your multiplier for a 5000 PSI tank TheLaughingMan!
Carbon Dioxide General Information-
Carbon dioxide (CO2) is different than high pressure air, whereas air is stored as a gas in your tank, carbon dioxide is actually a liquid as well as a gas in your tank. This means that carbon dioxide is not subject to the ideal gas law since any pressure lost is replaced by further evaporation of the carbon dioxide liquid in your tank. Ever wonder why your tank gets really cold after shooting a lot? Ever wonder why you cool down when you sweat? Both cases work on the same principal. In order for your body to cool down it releases sweat which absorbs the heat from your body and evaporates. See, in order for a molecule of something to evaporate, in this case sweat or carbon dioxide, it needs to speed up. And by absorbing heat it speeds up and after a certain amount of heat the molecule breaks free and goes from being a liquid to being a gas. When that heat is spent to create the gas molecule the body or tank gets colder.
Here is a graph that shows the % fill to pressure:
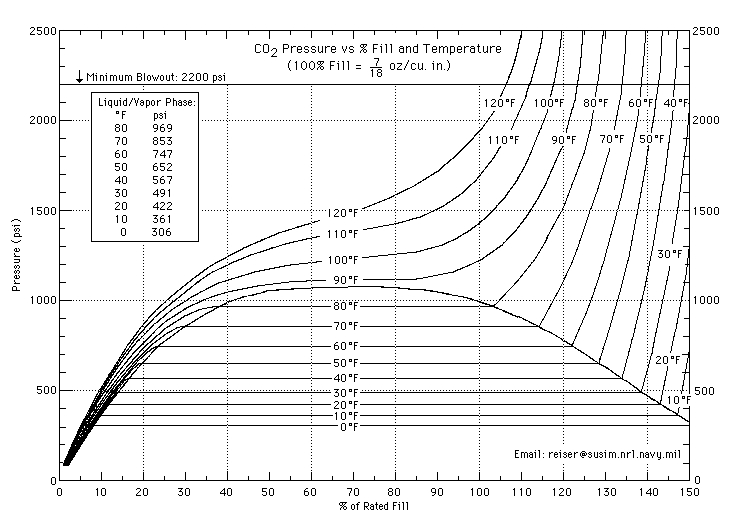
A "full" tank contains about 34% liquid CO2. If it is filled any more, the CO2 will become very sensitive to temperature changes, with a small increase in temperature causing a large increase in pressure. You can see from the above graph that after 70% of a rated fill the pressure spikes dramatically with an increase in temperature. This is a dangerous situation which is avoided by only partially filling the CO2 bottle.
One cubic inch of water weighs 0.577 oz and the specific gravity of liquid CO2 is 1.977 gm/cc so one ounce of liquid CO2 has a volume of 0.877 cubic inches. CO2 bottles generally have a full-fill to volume ratio of about 2.57 cubic inches per ounce of CO2, so that one ounce of CO2 will take up 0.877/2.57 = 34% of the total volume of the bottle.
The figure of 68% is often quoted as the volume of liquid in a full bottle, but this error probably stems from translating "ounce" into volume using water as the standard. Water is 1.00 gm/cc, or about half the density of liquid CO2 so that if a CO2 bottle is filled to its rated capacity with water, it will be 68% liquid by volume.
Here is a formula that will find the pressure in a CO2 tank:
P = (314.04 psi)(2.72)(T/69.64 *F)
Note: this equation will work well with values between 0-80 *F.
How do I find the pressure in a CO2 tank at a given temperature?
I will solve for 72 *F (room temperature).
1. First convert all known values to metric with the help of Google:
None!
2. Plug the numbers into the equation and solve for the unknown:
P = (314.04 psi)(2.72)(72 *F/69.64 *F)
P = 883 psi!
[b]
